Resources included (11)
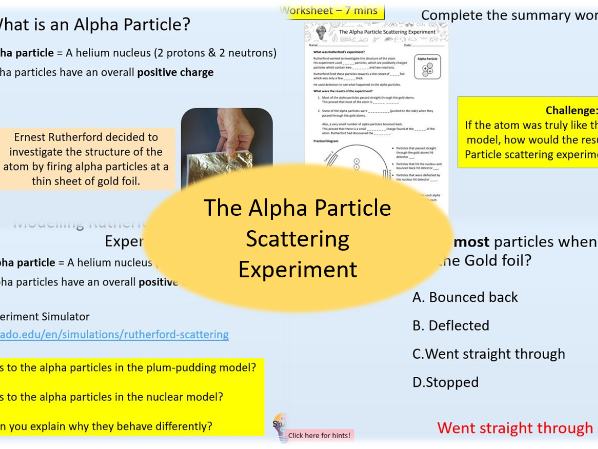
The Alpha Particle Scattering Experiment (History of the Atomic Model: Lesson 2)
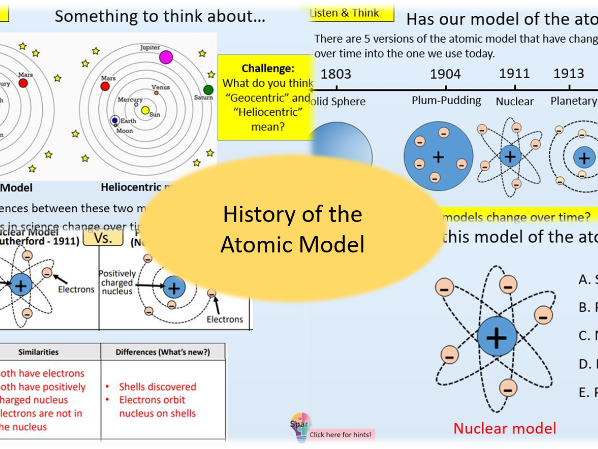
History of the Atomic Model
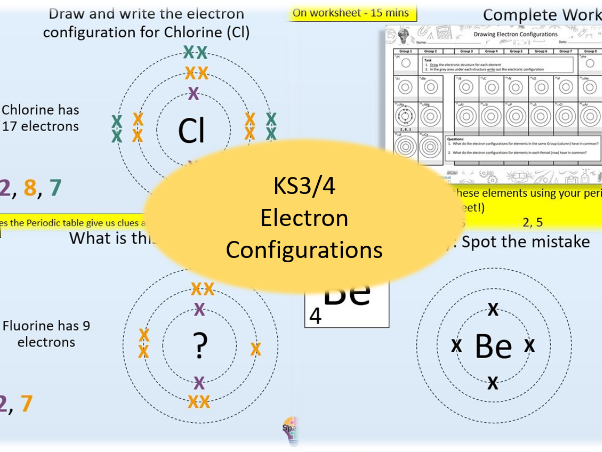
Electron Configurations (2,8,8 format - GCSE Chemistry)
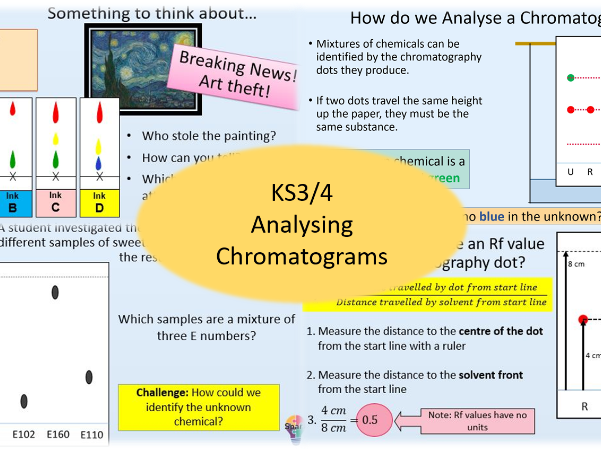
Analysing Chromatograms
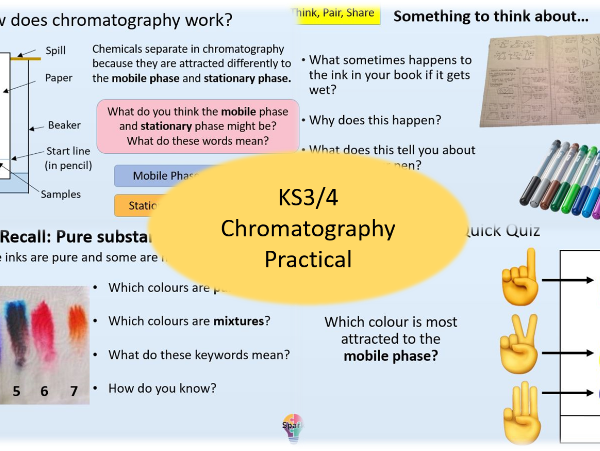
Chromatography (Including Practical)
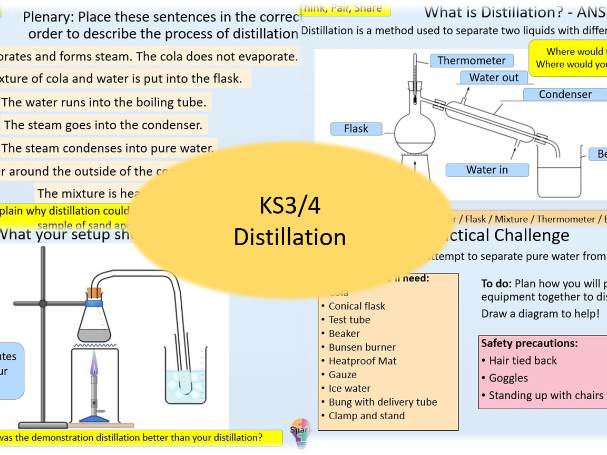
Distillation
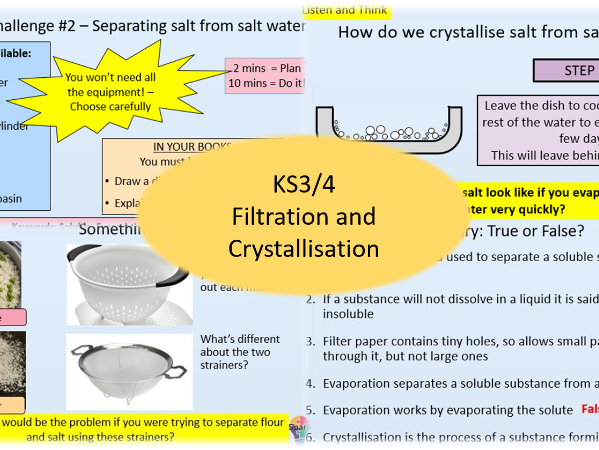
Filtration and Crystallisation

Counting Subatomic Particles

Structure of the Atom

Elements and Compounds

Chemical Mixtures
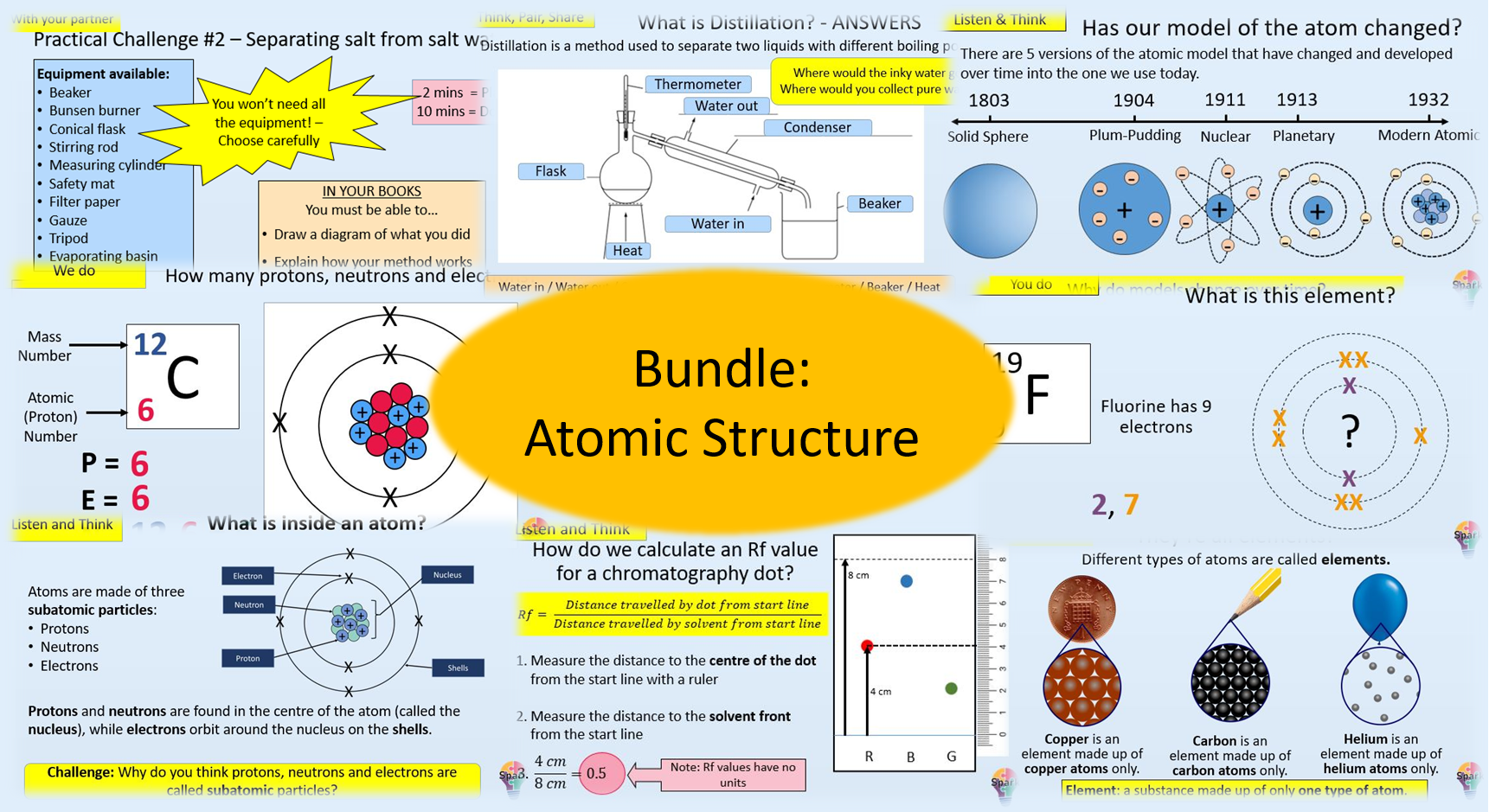
This topic bundle is designed as part of a bridging Year 9, covering content from AQA GCSE Chemistry Topic 1 “Atomic Structure and the Periodic Table”. which help students build strong foundations of knowledge they will need to succeed in GCSE science.Â
This bundle contains the following lessons:
- Elements and Compounds
- Mixtures
- Filtration and Crystallisation
- Distillation
- Chromatography Practical
- Analysing Chromatograms
- Structure of the Atom
- Counting Subatomic Particles
- Electron Configurations
- History of the Atomic Model
- The Alpha Particle Scattering Experiment
This bundle is a complete topic pack containing all powerpoints, student worksheets, stretch and challenge tasks, and answer sheets for the Chemistry topic “Atomic Structure”. It also contains many student-led activities on the powerpoints, plenary activities, a variety of AfL activities, and reading and literacy tasks.
Lesson objectives:
- Know what “element”, “compound”, “molecule” and “atom” mean
- Identify elements, compounds, molecules and atoms in particle diagrams and chemical formulae
- State what a mixture is
- Describe particle diagrams of mixtures in terms of elements and compounds
- Give limitations of using different models to represent mixtures
- Know what “soluble” and “insoluble” mean
- Identify mixtures that can be separated using filtration and crystallisation
- Describe and explain the process of filtration and crystallisation to separate a mixture
- State what evaporate and condense mean
- Identify mixtures that can be separated using distillation
- Describe and explain the process of distillation to separate a mixture
- Identify the mobile and stationary phase in paper chromatography
- Describe how to use chromatography to separate the substances in a mixture
- Explain why different substances travel different distances in chromatography
- Carry out a chromatography practical to investigate the composition of inks
- Analyse the composition of a mixture using a chromatogram
- Calculate the Rf value for a chromatogram spot
- Identify unknown chemicals using Rf values
- Describe what a subatomic particle is
- Describe the structure of an atom in terms of subatomic particles
- Give the positions, relative mass and charge of the three subatomic particles
- Know what the mass number and proton/atomic number tell us about an element
- Count the number of protons, neutrons and electrons found in elements
- Explain why the number of protons and electrons are always the same in atoms
- State how many electrons sit in each shell of an atom
- Draw and write the electron configuration for the first 20 elements of the periodic table
- Identify elements from their electron configurations
- Identify and name the different historical models of the atom
- State the major discoveries about the structure of the atom and the scientists associated with them
- Compare and contrast features of different models of the atom
- Describe the alpha particle scattering experiment and its results
- Explain the results of the alpha particle scattering experiment and what it proved about the structure of the atom
Resource contains:
- Lesson powerpoints for each lesson (.pptx)
- Student worksheets (PDF)
- Student worksheet answers (PDF)
Lessons contain:
- Interactive lesson powerpoints for all lessons with full answers
- Teacher delivery instructions and tips in Powerpoint notes sections
- Stretch and challenge tasks throughout
- Independent student-led activities including reading comprehension
- Explicitly taught scientific literacy
- A variety of AfL tasks throughout
- Plenary tasks and quizzes for each lesson
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.