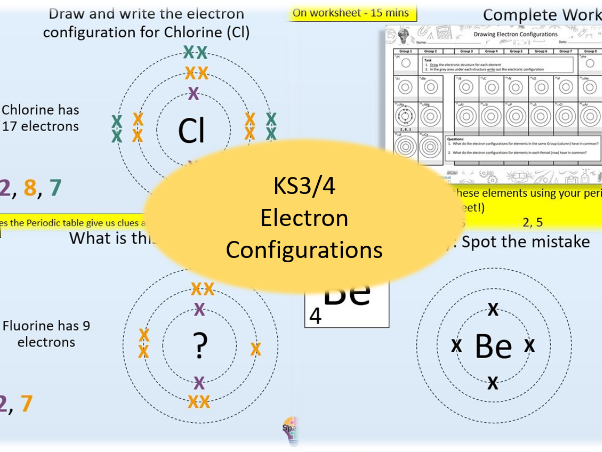



In this lesson students will learn how to arrange electrons on the shells around the atom, the 2,8,8 rule and how to predict an atom’s electron configuration using the element’s position on the Periodic table.
This lesson uses structured scaffolding, colour-coding and AfL techniques to help students grasp this vital chemistry skill.
This lesson is the ninth lesson in the “Atomic Structure” topic. This topic is designed to act as a “bridging topic” between KS3 and KS4 chemistry.
Lesson Objectives:
- State how many electrons sit in each shell of an atom
- Draw and write the electron configuration for the first 20 elements of the periodic table
- Identify elements from their electron configurations
Resource contains:
- Lesson Powerpoint (pptx.)
- Student Worksheet (PDF)
- Student Worksheet Answers (PDF)
Lesson contains:
- Interactive powerpoint including teacher delivery notes and answers throughout
- AFL activities
- Plenary task
- Stretch and challenge tasks throughout
- “I do, we do, you do” scaffolded modelling
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.