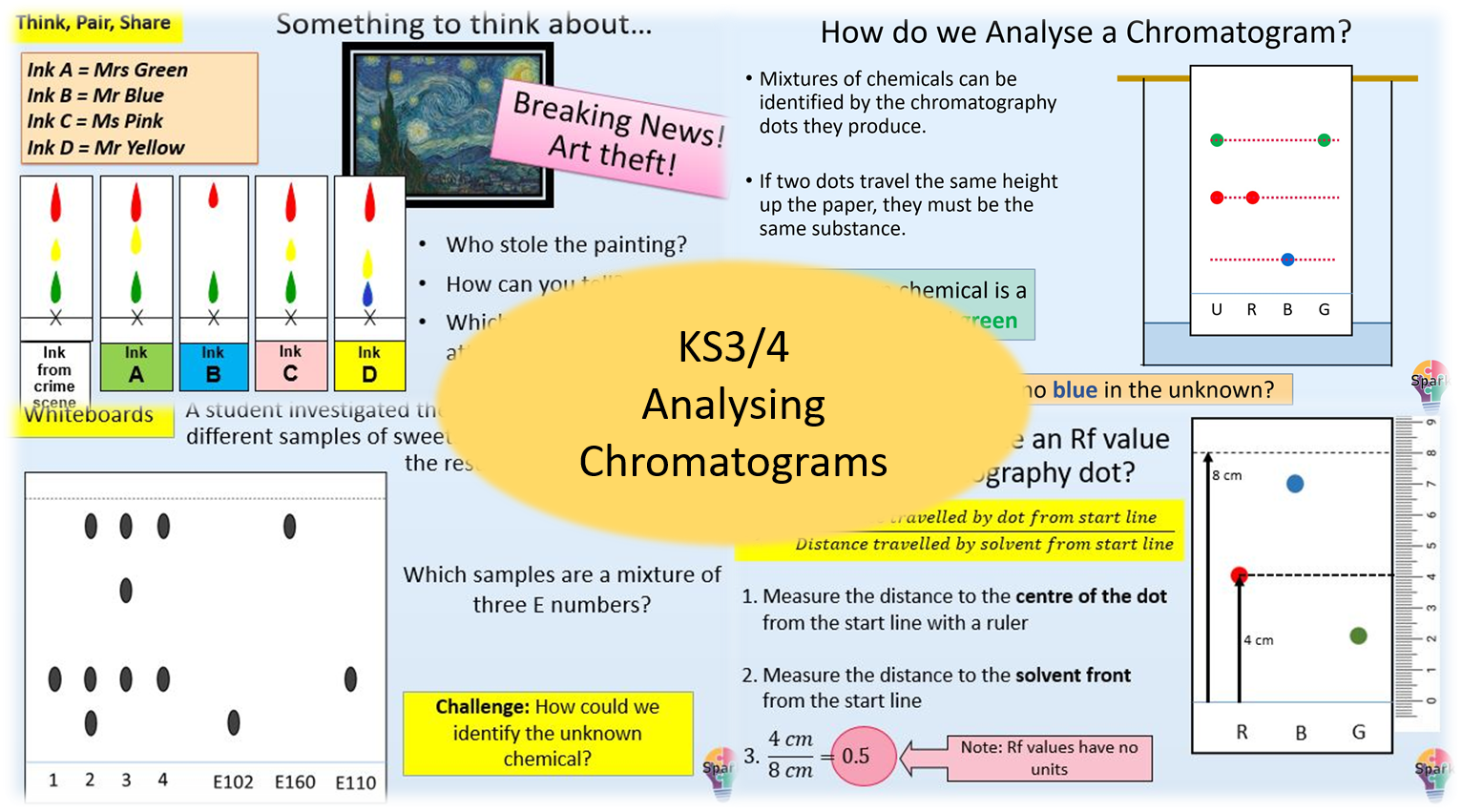


In this lesson students will learn how to read and analyse chromatograms, as well as calculate Rf values.
This lesson will also allow students to see some of the applications and uses of chromatography, as well as identify common mistakes and misconceptions.
This lesson is the sixth lesson in the “Atomic Structure” topic. This topic is designed to act as a “bridging topic” between KS3 and KS4 chemistry.
NOTE: This is a follow-on lesson from the previous lesson on “Chromatography”, and will require some background understanding from that on how chromatograms are made.
Lesson objectives:
- Analyse the composition of a mixture using a chromatogram
- Calculate the Rf value for a chromatogram spot
- Identify unknown chemicals using Rf values
Resource contains:
- Interactive powerpoint including teacher delivery notes and answers throughout
- AFL activities
- Plenary task
- Stretch and challenge tasks throughout
- Explicit teaching of scientific vocabulary
- Explicit teaching of common misconceptions and mistakes
Lesson contains:
- Lesson Powerpoint (.pptx)
- Student Worksheet (PDF)
- Student Worksheet Answers (PDF)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.