
What are concordant titres? Which substance goes in the conical flask and which substance goes in the burette? A level chemistry titration method is what you’re after? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – This slide explains the objectives of a titration
Slide 4 – A list of key words and their meanings (equivalence points, end point, titre and concordant titres)
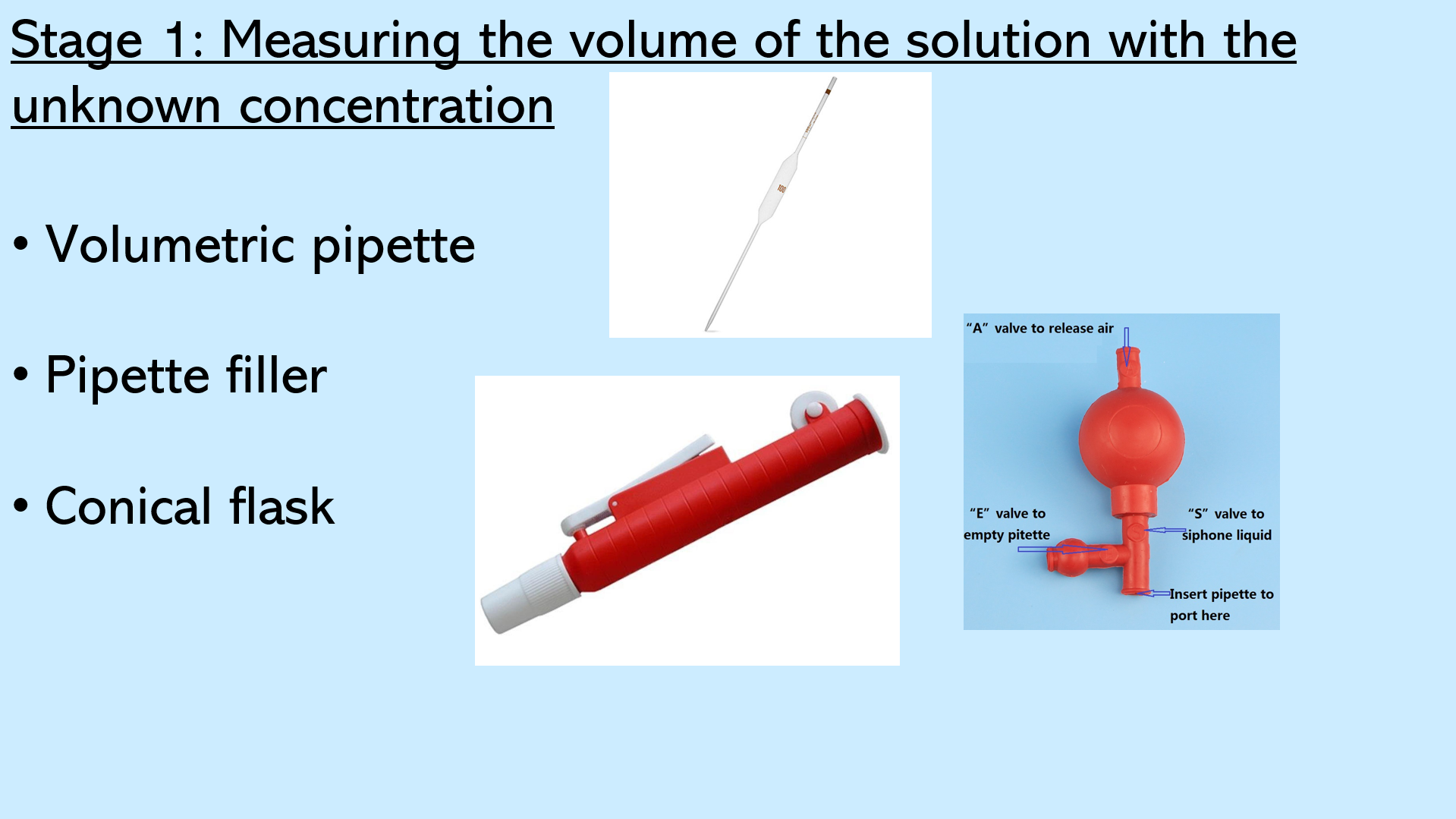
Slides 5 - This slide goes through the details of stage one of a titration, which includes measuring the volume of the solution with the unknown concentration
Slide 6 - This stage is #2 in a titration which is adding the indicator to the solution of unknown concentration, details or phenolphthalein and methyl orange
Slides 7 – 8: these slides give the colours of phenolphthalein and methyl orange in acids an alkalis, respectively
Slides 9 -10: stage 3 which is measuring the volume of the solution with the known concentration also known as the standard solution and students are given a useful hack so remember where the standard solution goes during a titration
Slides 11 - 13 stage 4 carrying out the titration and reading the burette, plus recording results. Print slides 12 to 13 for the students so they can have the sample table
Slides 14 – 18: Introduces the students to the practical that they will do which sulfamic acid as a standard solution and sodium hydroxide as the solution of unknown concentration, chemicals are listed along with apparatus and the method. Slide 18 lists some titration top tips which could be useful to share with your students
Slide 19: learning pit stop - two questions which students can answer if they finish the experiment early, once they have calculated the concentration of the unknown sodium hydroxide solution. Answers animate onto the screen as you click
Slide 20 - What was the concentration of your sodium hydroxide solution? A chance for pooling of results and discussion
Slides 21 – 28: Exam questions with mark scheme answers (included with the purchase of this resource)
If you have a positive experience with the resource, please leave a positive review! This really helps promote our store! Thanks!
Something went wrong, please try again later.
This is a really well-structured lesson. The flow of activities seems very logical and guides students through the topic effectively.
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.