
What is enthalpy? What is the difference between heat energy and temperature? What is enthalpy change of reaction? What are standard conditions? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Introduction to the laws of thermodynamic
Slide 4 – focus on the first law (conservation of energy)
Slide 5 – Chemical energy definition and potential energy definition
Slide 6 – Heat energy definition
Slide 7 - learning pit-stop to check students’ learning. A series of questions of increasing difficulty, with the stretch and challenge (S+C) being the hardest. Answers animate onto the screen when you click
Slide 8 – Enthalpy information, including definition and what exactly is meant by surroundings
Slide 9 – meaning of exothermic and endothermic, in terms of system and surroundings
Slide 10 – 13: Classifying activity – students must classify the chemical and physical changes as either exothermic or endothermic. Print slides 11 – 12 (hidden)
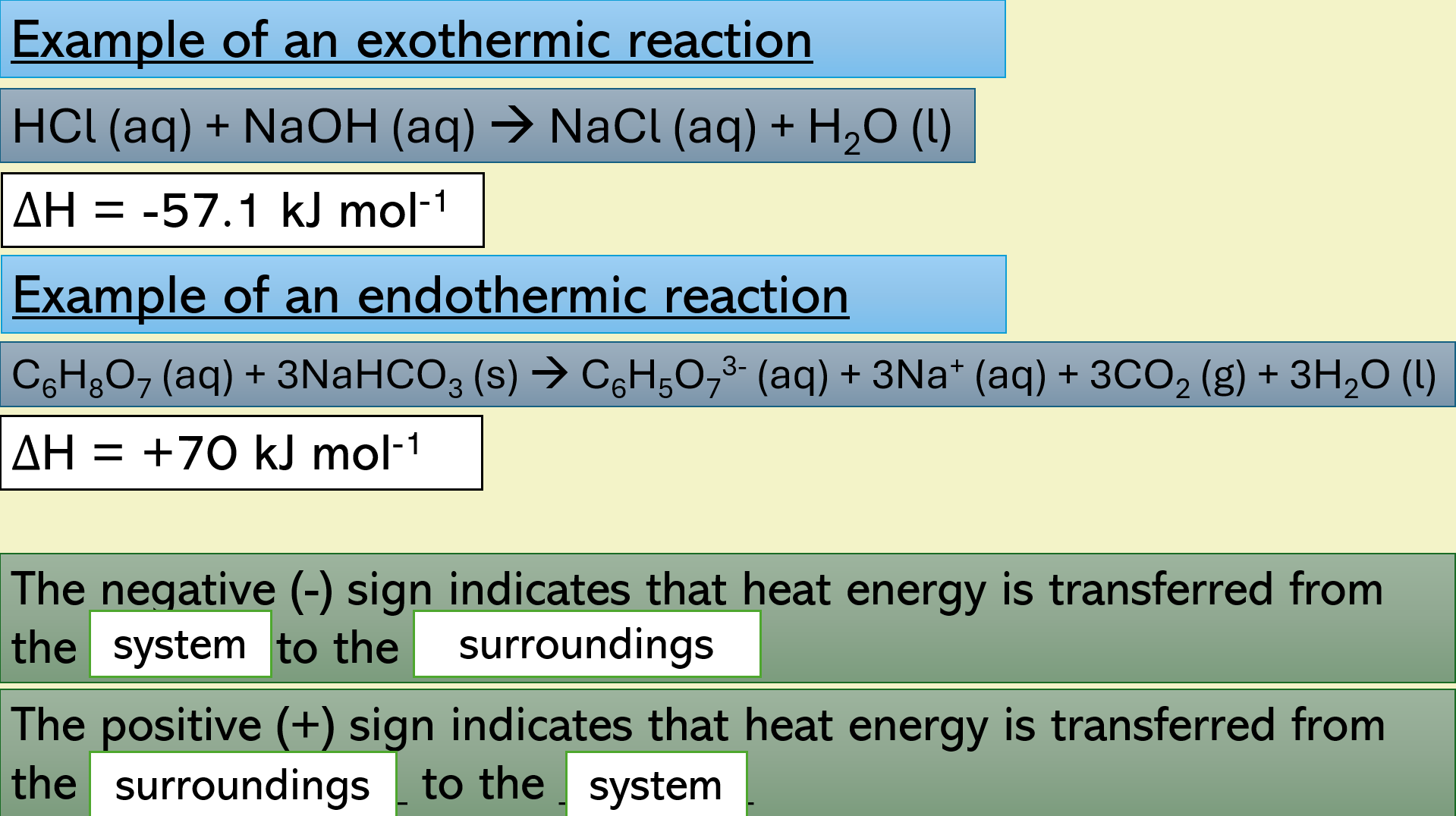
Slide 14 – example of an exothermic and an endothermic reaction, showing the negative and positive delta H, and explaining what this means
Slide 15 – definition of standard enthalpy change of reaction
Slide 16 – explanation of standard conditions
Slide 17 – Applied Learning Time (ALT): series of questions to provide students with the opportunity to embed their learning. Answers animate on the screen as you click
If you have a positive experience with the resource please leave a positive review! This really helps promote our store! Thanks!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.