

How are alcohols classified? How do alcohols react with PCl5, red phosphorus and iodine, potassium bromide and 50% sulfuric acid? Alcohol to alkene? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Alcohol as a homologous series with a general formula
Slides 4 – 7: Students are given a table with structures and IUPAC name as the headings. There are some blank cells in the table which the students must complete. Print slides 5-6 so that students can have the table in front of them and not waste time copying it. On slide 7 there is a challenge question in which students are asked to draw some skeletal formula for some alcohols
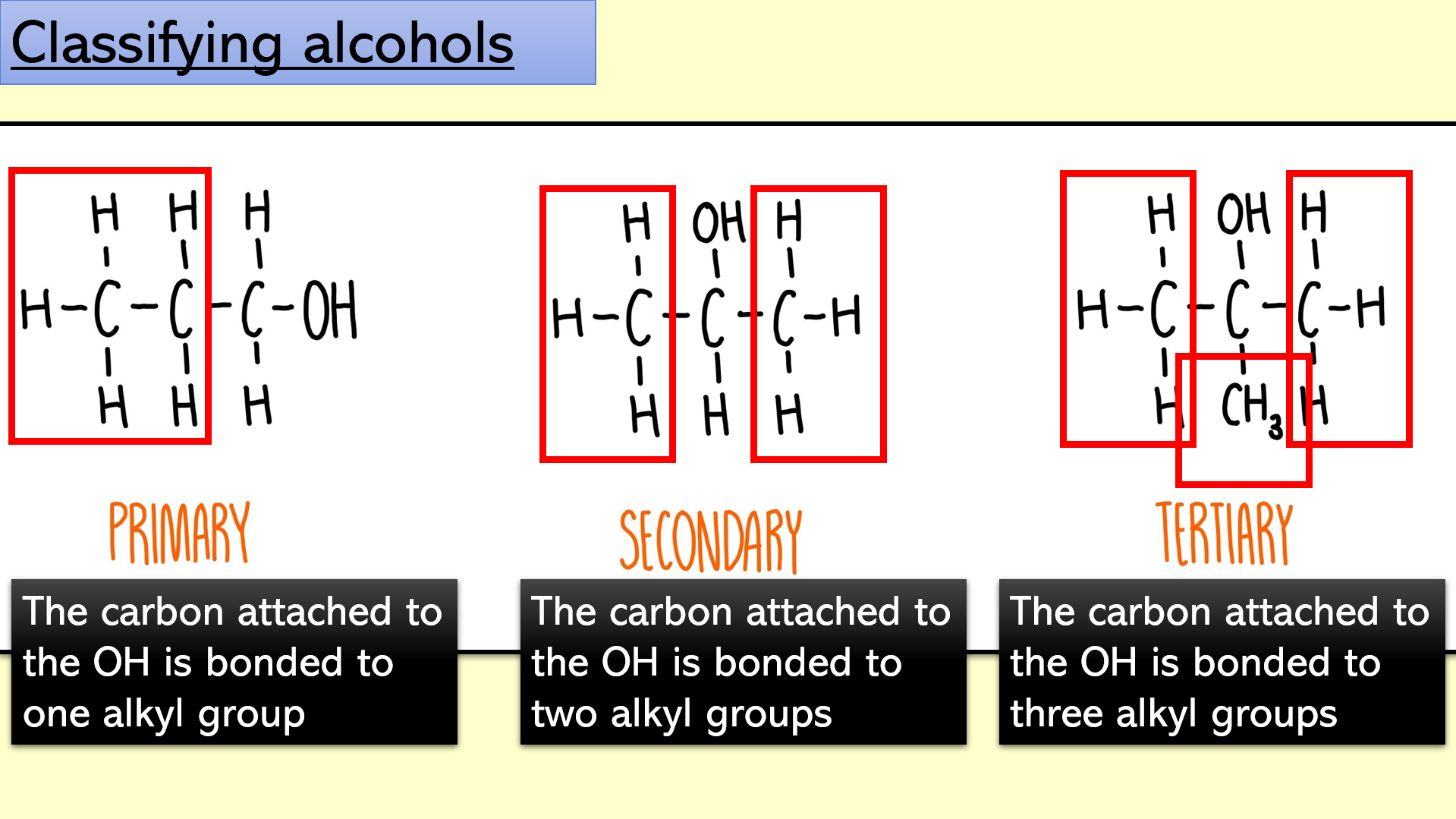
Slide 8 - classification of alcohols
Slide 9 - an overview of the reactions of alcohols by way of a spider diagram
Slide 10 - combustion of alcohols, with the chemical equation for the complete combustion of ethanol
Slide 11 - chlorination of alcohols using phosphorus pentachloride. Explanation is given as to how this reaction can be used as a qualitative test for evidence of the hydroxyl group
Slide 12 - bromination of alcohols using potassium bromide and 50% concentrated sulfuric acid. Conditions are given, along with example chemical equations
Slide 13 - iodination of alcohols using red phosphorus and iodine. Conditions are given, along with example chemical equations
Slide 14 - dehydration of alcohols to form alkenes using phosphoric acid as a catalyst.
Slides 15 – 22: Exam questions with mark scheme answers (included with the purchase of this resource)
Learning outcomes:
- Define and classify alcohols based on structure
- Write balanced equations for combustion and halogenation
- Identify reagents and conditions for alcohol synthesis and elimination
- Apply knowledge in exam-style contexts
- This lesson provides a clear and engaging overview of alcohol chemistry—ideal for exam preparation, core teaching, or practical theory consolidation.
If this resource supports your teaching, please consider leaving a positive review—it really helps Lifeboat Teachers continue creating ready-to-use lessons for busy educators!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.