

What is the difference between oxidation state and oxidation number? What are the rules for assigning oxidation states? What is disproportionation? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
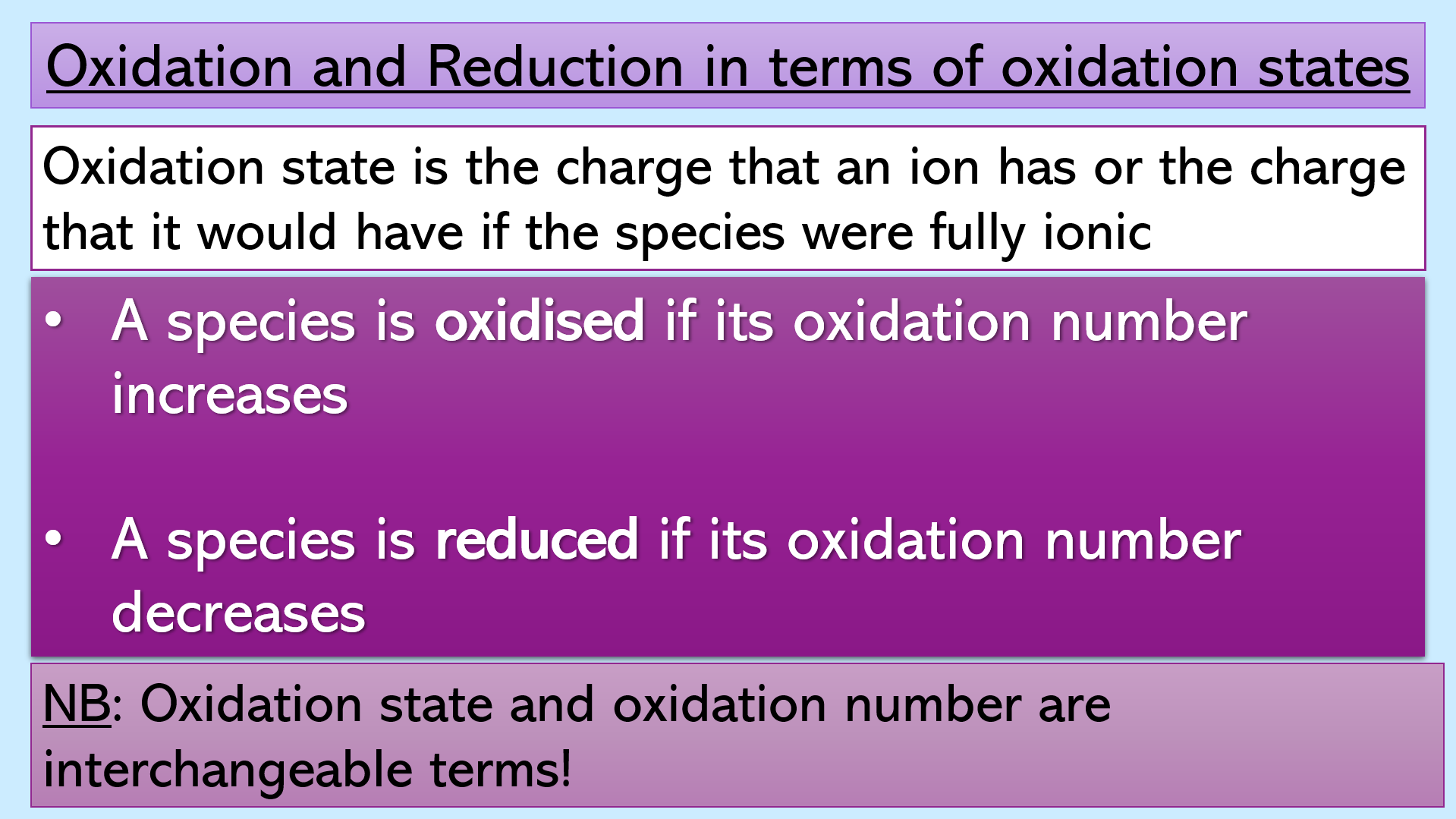
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Introduction to the three ways to describe redox reactions (in terms of oxygen/hydrogen, in terms of electron transfer and in terms of oxidation number)
Slide 4 – Definition, with example, of oxidation and reduction in terms of hydrogen
Slide 5 – Cold-call question
Slide 6 – Word fill exercise about oxidising and reducing agents
Slide 7 – IGCSE recap on oxidation and reduction in terms of electrons
Slides 8 – 10: Worked example to work out which species has been oxidised and which has been reduced using an ionic equation
Slide 11 – Introduction to the concept of oxidation state and how it can be used to work out if a species has been oxidised and reduced.
Slides 12 – 18: Some of the rules regarding assigning oxidation states. Print slides 13, 14, and 18 for students
Slides 19 – 21: A series of worked examples, showing students how to work out the oxidation states
Slide 22 – Independent practice for students to complete in silence
Slide 23 – Answers
Slide 24 – Challenge question for the most able
Slide 25 – Disproportionation introduced to students with an example
Slide 26 – definition of disproportionation
Slides 27 – 34: Exam questions with mark scheme answers (included with the purchase of this resource)
If you have a positive experience with the resource, please leave a positive review! This really helps promote our store! Thanks!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.