

What is rate of reaction? How is it measured? What does initial rate mean? This detailed and fully animated A Level Chemistry lesson answers all of that and more with engaging visuals, structured mini plenaries, and exam-style questions.
Created for Edexcel International A Level Chemistry Unit 2 (WCH12), this resource is also ideal for AQA, OCR, and other major UK specifications. It’s designed to help Year 12 students build a strong understanding of reaction kinetics, data interpretation, and particle theory—all essential for exam success.
PowerPoint includes:
Slide 1: Title + 5-minute starter grid (last week / last lesson / today / next lesson)
Slide 2: Learning objectives
Slides 3–4: Introduce rate through real-world data (crime rate analogy)
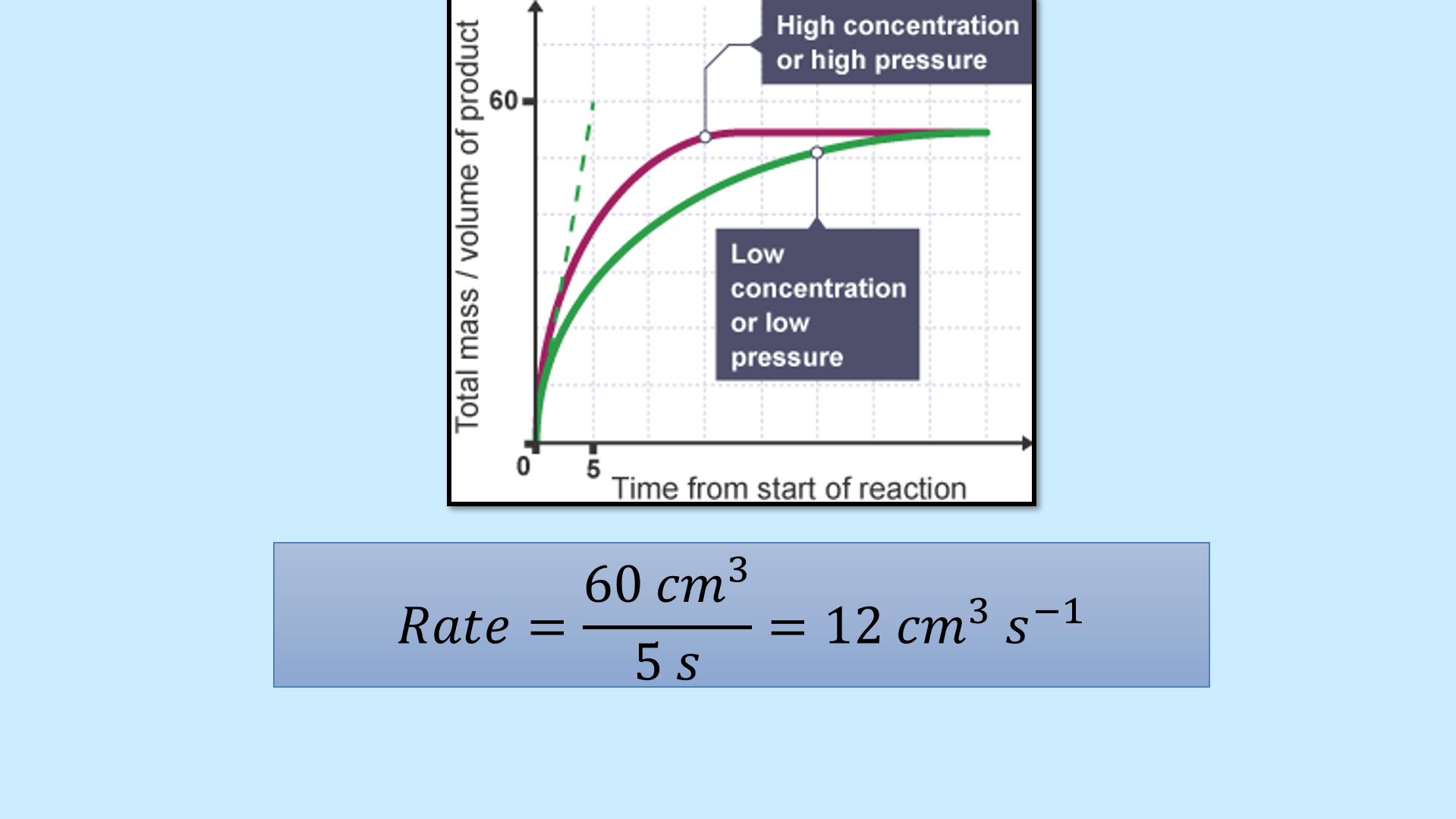
Slides 5–8: Track rate via changes in reactants/products in a CaCO₃ + HCl reaction
Slides 9–11: Tangent graph task to calculate rate at a point
Slides 12–13: How to calculate initial rate from a curve
Slides 14–18: Collision theory using particle diagrams + activation energy explained
Slides 19–20: Why not all collisions cause reactions
Slides 21–22: Stretch/challenge: covalent bonds and energy in organic reactions
Slides 23–24: Exam questions with answers
Key learning outcomes:
Define and calculate rate of reaction
Interpret concentration–time graphs
Use tangents to estimate rate at a specific time
Understand molecular collisions, orientation, and energy requirements
Build exam technique and confidence
Ideal for classroom teaching, flipped learning, cover lessons, or exam prep. Fully animated with answers that appear on click—no extra prep needed.
If this resource supports your teaching, please consider leaving a positive review—it helps Lifeboat Teachers keep creating time-saving lessons for busy educators.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.