
What are analytical techniques? What is the M+1 peak? What is a fragmentation pattern? What are common m/z values and possible ions? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Recap questions on mass spectrometry from Topic 2. Answers animate on to the screen when you click
Slide 4-mass spectrum of a polyatomic molecule. A label diagram to remind students of how the mass spectrometer works
Slide 5 - mass spectrum of ethane is displayed on the board for you to discuss with the students
Slide 6 - the definitions of molecular ion peak and M+1 peak
Slide 7 - explanation of the concept of fragmentation
Slide 8 – a visual representation of fragmentation of an ethane molecule
Slide 9 - equations to represent fragmentation of an ethane molecule
Slides 10 – 14: The mass spectrum of butane. Certain peaks have been labelled to focus the students’ attention and, on later slides, these peaks are matched with their corresponding ions. Prints slides 12 - 13 so that students can annotate the mass spectrum of butane as you go through it
Slide 15 – learning pit stop. A series of questions for students to answer in their copy books. Answers animate on the screen when you are ready to review
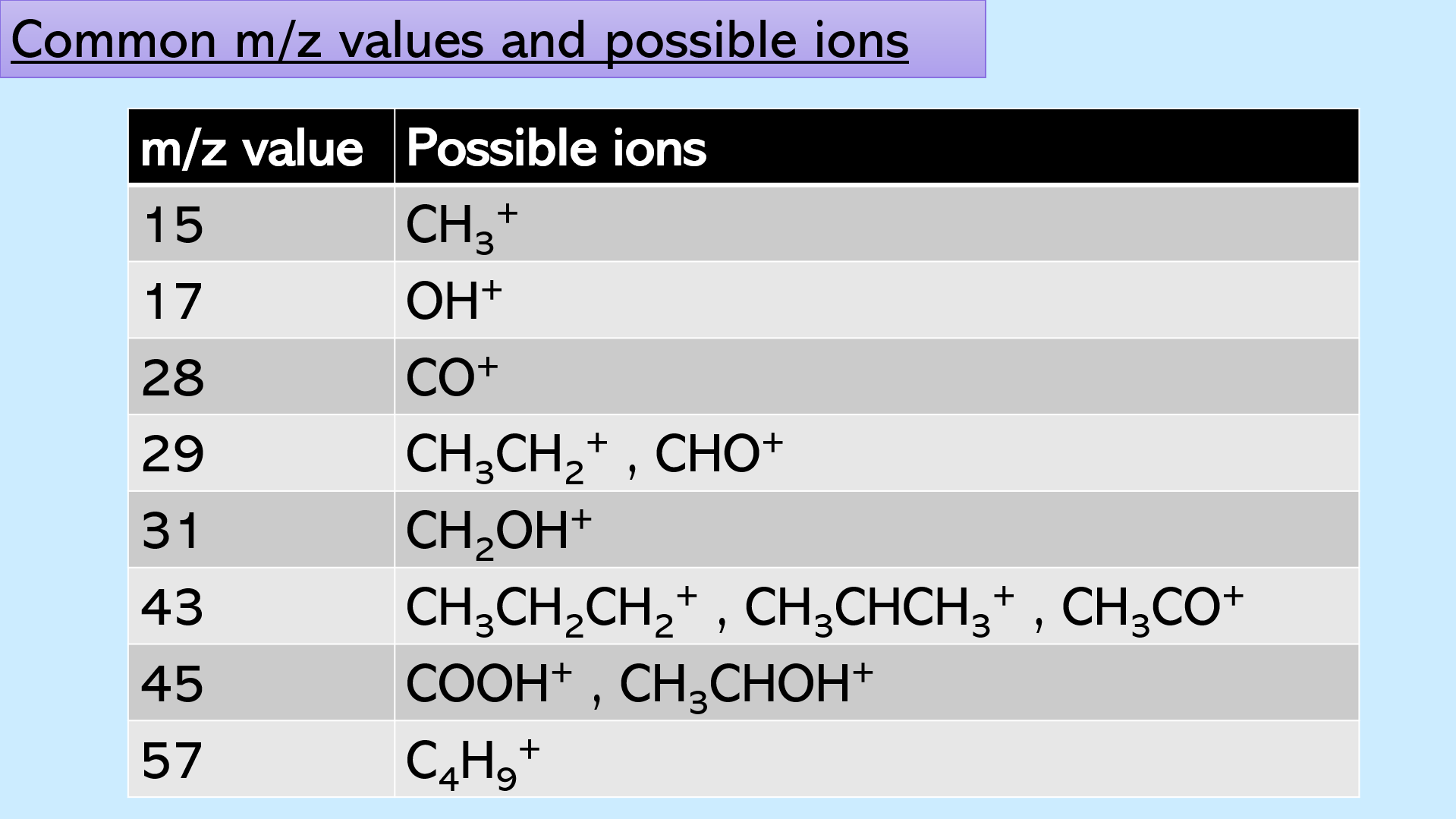
slides 16-18: a table with common M/Z values and the possible, corresponding ions. Print slides 17 – 18 for the students so that they can glue the table into their copy books
Slides 19 - 20: students are given a mass spectrum for an isomer of C2H6O and must answer a series of questions, such as, identify the molar mass of the molecular ion, identify the fragment that gives rise to the peak at M/Z 29, etc
Slides 21- 25: Worked example 1: Two compounds, A and B, have the molecular formula C3H6O. Their simplified mass Spectra are shown. Can you deduce the structure of each one? Detailed working out is included and animates on the screen as you click
Slides 26 – 30: Worked example 2
Slides 31 – 37: Exam questions with mark scheme answers (included with the purchase of this resource)
If this lesson saves you planning time, please consider leaving a positive review. Your feedback helps Lifeboat Teachers continue to support time-poor educators!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.