A full 1 hour lesson designed for a mixed ability year 8 class.
This is the 3rd lesson in “Chapter 3 - Metals and other materials" from Activate 2, Chemistry. This lesson is on the page named ‘Reactivity series’, but the main focus of the lesson is on the reactions of metals with water.
This lesson should be suitable to teach to any KS3 Chemistry class, even by those where Chemistry is not their specialism.
This lesson (and all lessons in this unit) is designed to be interactive and engaging, with plenty of real world examples and independent tasks.
This lesson contains two guided practical demonstrations (calcium granules in cold water, and reacting magnesium with steam) complete with risk assessments.
From this lesson, students should be able to:
- Recall that in a reaction between a metal and water, a metal hydroxide and hydrogen gas are formed
- Understand that not all metals react with water, and metals react differently with water depending on their reactivity
- Name the metal hydroxide formed when a specific metal reacts with water
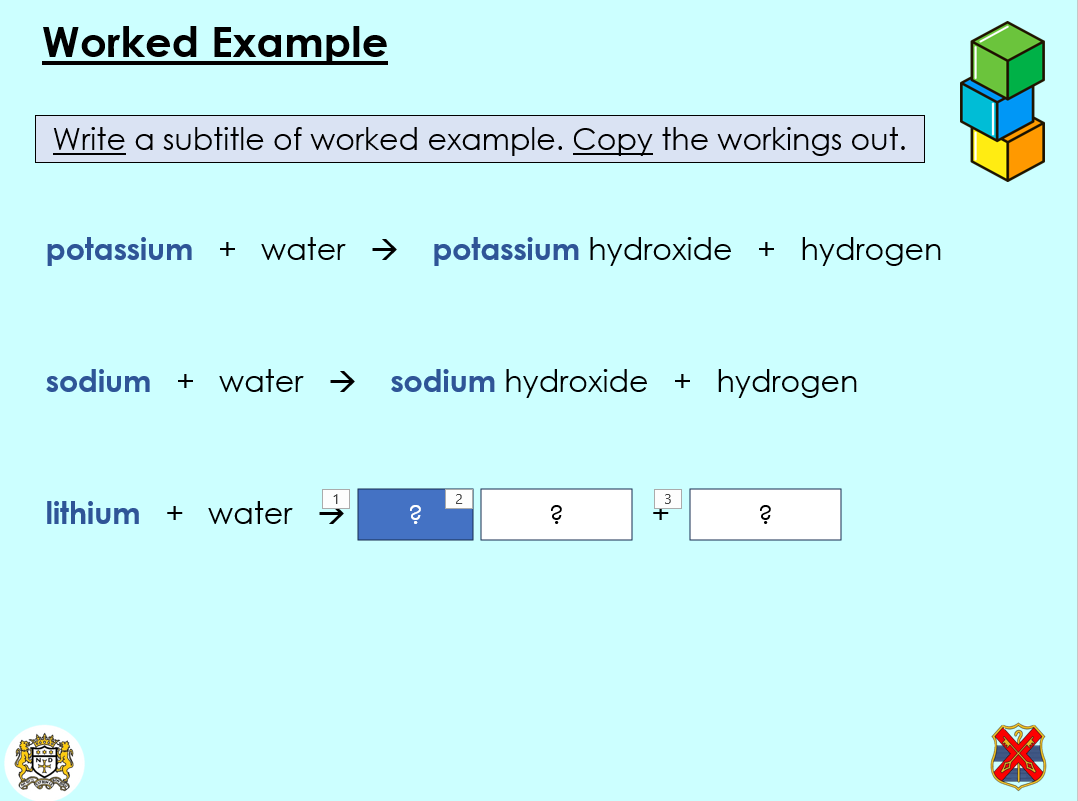
- Write word equations for the reactions of metals with water
- Use the reactions of metals with water to determine the reactivity series
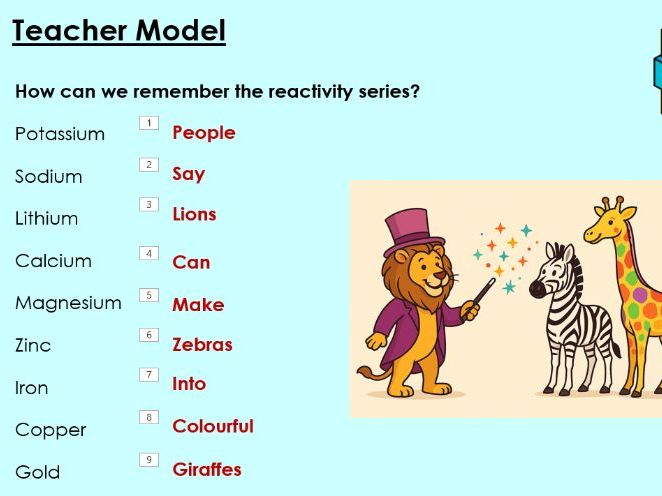
- Recall the reactivity series for these metals: Potassium, sodium, lithium, calcium, magnesium, zinc, iron, copper, gold.
All of my lessons contain:
- A 5-in-5 retrieval-style starter
- An interesting lesson hook, careers link, or retrieval practice to start the lesson
- Teacher input slides with dual coding and reduced cognitive load
- Teacher models
- Regular ‘check for understanding’ slides, such as hand signals quizzes and whiteboard quizzes
- Regular student independent practice slides, with optional scaffolds, challenges and answer slides
- A plenary task
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.