
Why does reactivity of group 1 metals increase down the group? How do alkali metals react with oxygen, water and chlorine? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – What is the difference between a chemical and physical property?
Slides 4 – 7: A table is presented to students with a range of different properties – students must sort the physical and chemical properties
Slides 8 – 9: 3 mark exam style question; students presented with a data table and asked to identify the trends
Slide 10 - a short video on the reaction of alkali metals with water
Slides 11 -18: word equations for the reaction of Group 1 metals with water - students must fill in the blanks. You can do this activity as a cold call a session
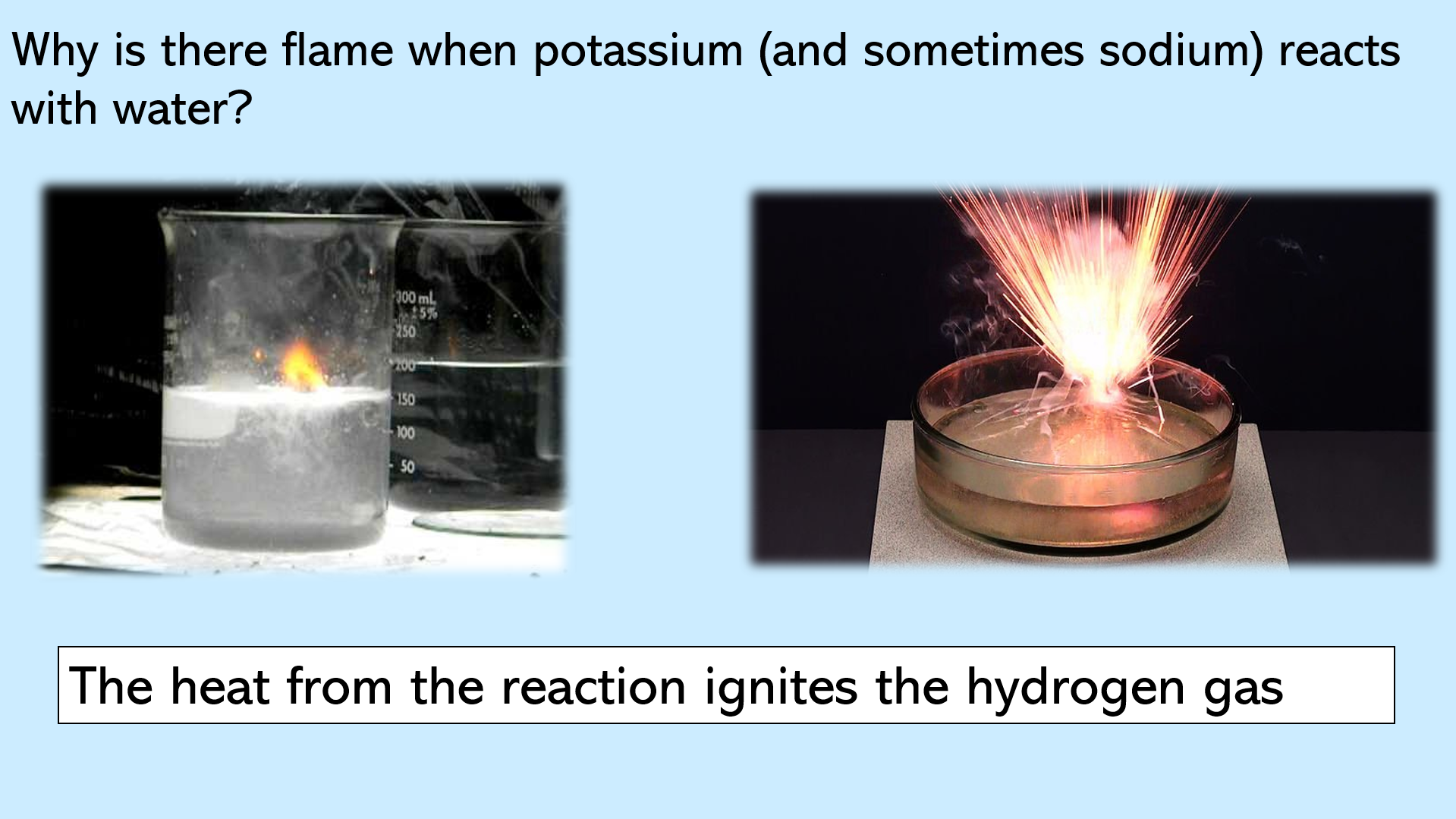
Slide 19 - why is there a flame when potassium (and sometimes sodium) reacts with water? This question is answered on this slide
Slide 20 - learning pit-stop to check students’ learning. A series of questions of increasing difficulty, with the stretch and challenge (S+C) being the hardest. Answers animate onto the screen when you click
Slides 21 – 22: why do the alkali metals get more reactive as you go down the group? This question can be cold called to the class and the answer is on slide 22
Slide 23: independent practice - A series of questions of increasing difficulty, with the stretch and challenge (S+C) being the hardest. Answers animate onto the screen when you click
If you have a positive experience with the resource, please leave a positive review! This really helps promote our store! Thanks!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.