

How do you dry an organic liquid with an anhydrous salt? How do you extract a solvent using a separating funnel? What is the difference between distillation and heat under reflux? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – A list of substances which can contaminate an organic liquid. This really sets the scene, as the lesson is about how to purify organic liquids
Slide 4 – List of techniques which organic chemists can use to purify organic liquids: heating under reflux, distillation, extraction with a solvent and drying with an anhydrous salt
Slide 5 – runs through the details of heating under reflux
Slide 6 – runs through the details of simple distillation, followed by advantages and disadvantages
Slide 7 - runs through the details of fractional distillation, followed by advantages and disadvantages
Slide 8 – a challenge is presented to help students think about extraction with a solvent. The challenge is as follows: you have a solvent with two compounds dissolved in it, one of which is your desired organic liquid. How can we separate the organic liquid, assuming that distillation is not an option? The solvent is chloroform and the substances are sodium chloride and caffeine
Slides 9 – 10: matching exercise. Students must arrange steps to solve the challenge and put them in the correct order. Answer given on slide 10
Slides 11 – 13: further details on how to ensure purity with extraction using a solvent and details on the choice of solvent. A diagram of the procedure is given on slide 13
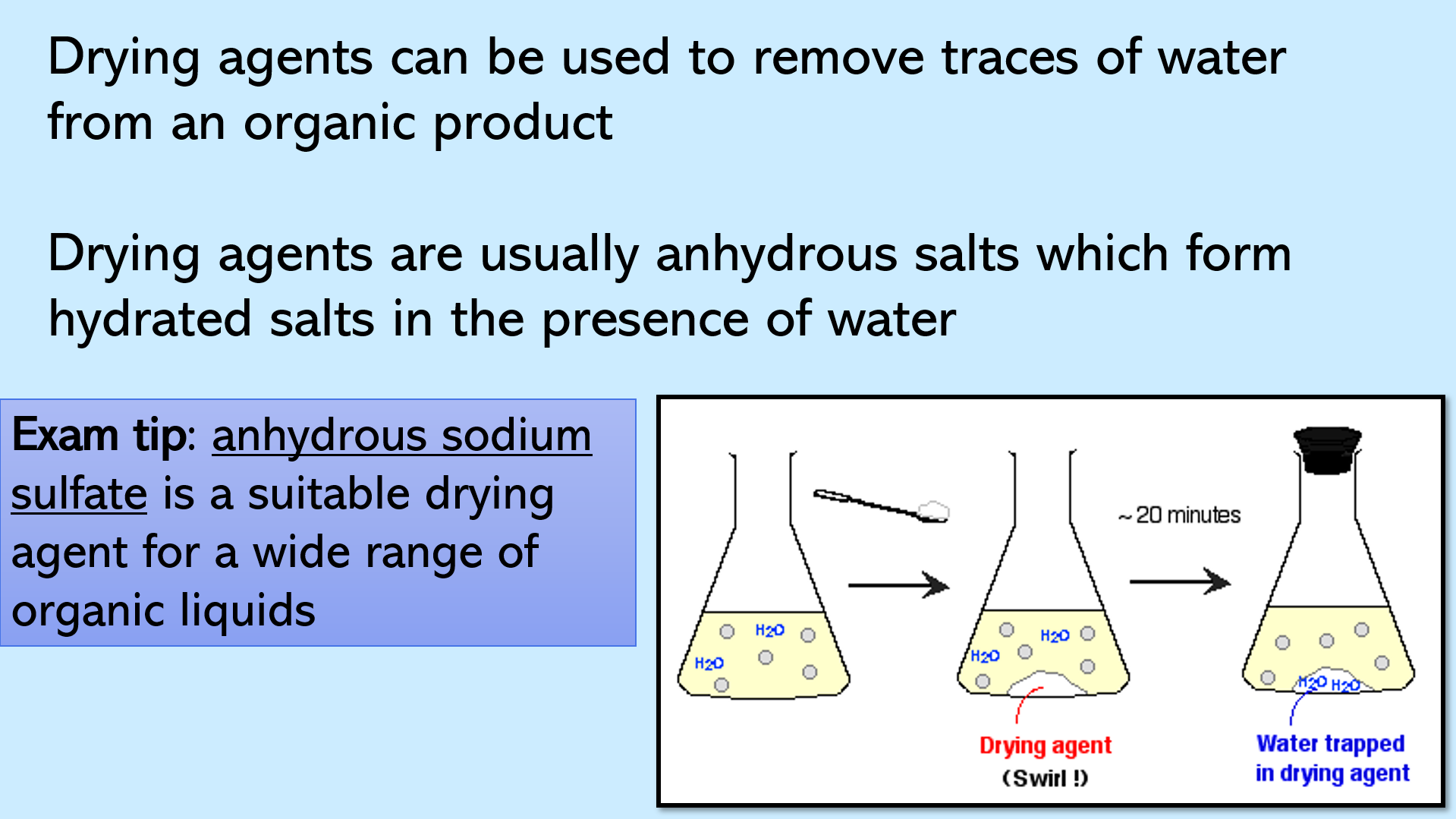
Slides 14 – 18: this section covers drying with an anhydrous salt. Includes tips on universally recommended drying agents, how they work, step-by-step, and a diagram showing decantation (this term may be new, especially for EAL learners)
Slide 19 – learning pit stop (mini plenary). Answers to all the questions animate onto the screen as you click
Slides 20 – 21: How do you ensure that a purified, organic liquid is pure? Advantages and disadvantages of using boiling point to this end are detailed in this section
Slides 22 – 27: Exam questions with mark scheme answers (included with the purchase of this resource)
If this lesson saves you planning time, please consider leaving a positive review. Your feedback helps Lifeboat Teachers continue to support time-poor educators!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.