

What is dynamic equilibrium? How do temperature changes affect position of equilibrium? What does equilibrium shift mean? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 12 A level lesson for Edexcel International Unit 2 – WCH12, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Explanation as to why some reactions are reversible and others are not
Slide 4-definition of dynamic equilibrium is presented to students on this slide
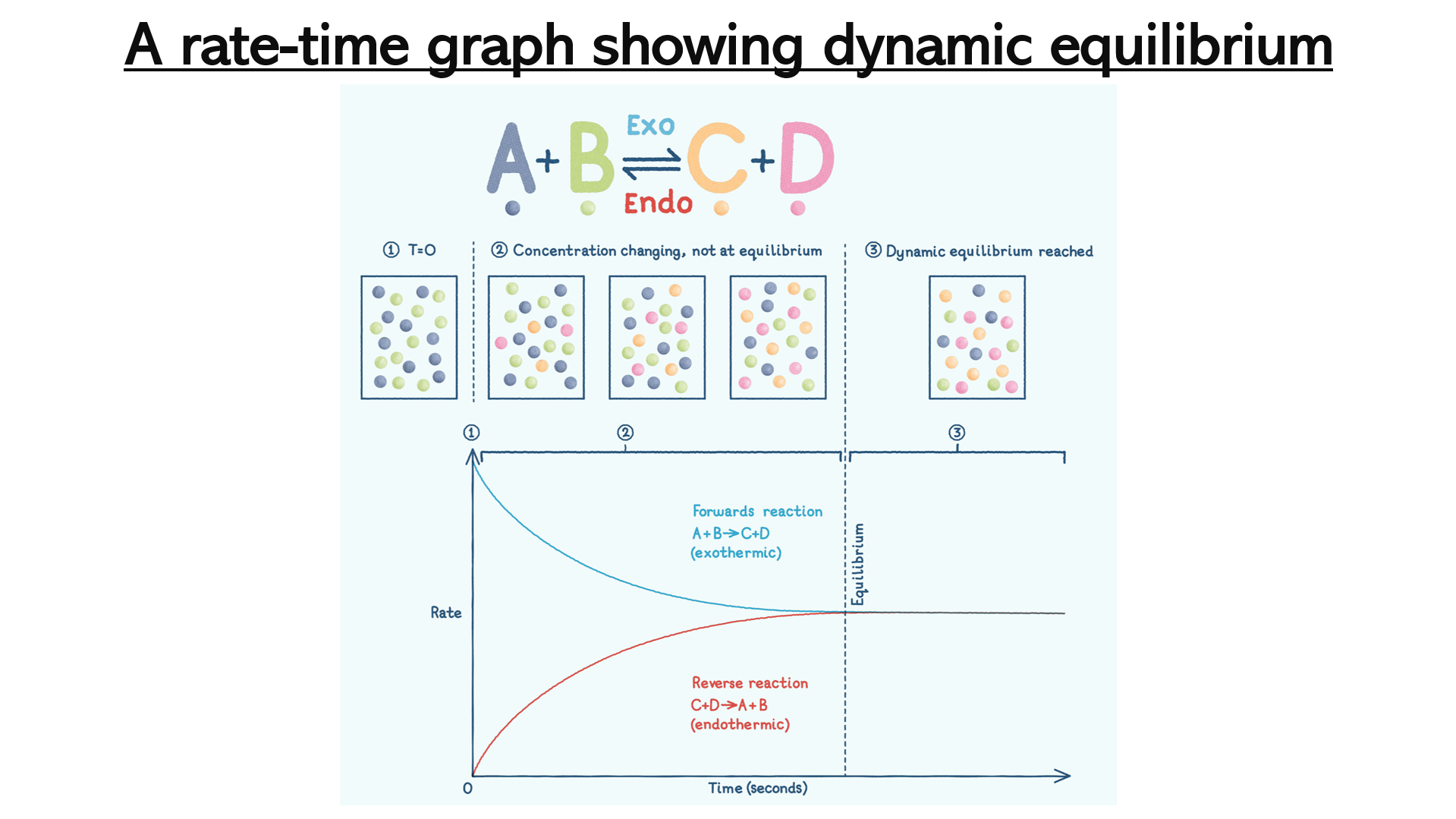
Slides 5 – 7: a rate time graph showing dynamic equilibrium. This graph will help students to appreciate that the rates of the forward and reverse reactions are the same. Print slide 6 - 7 so that students can have a copy of the graph to glue into their books.
Slides 8 - 9: dynamic equilibrium can only be reached in a sealed container. A visual of iodine vapour and died in crystals in an opening close system helps to reinforce this concept.
Slides 10 - 13: mini plenary exam questions (included with this resource) to allow students to reconsolidate their learning. Mark scheme answers animate onto the screen as you click when you are ready to review with the class.
Slides 14- 17: a word fill exercise to help students gain confidence using the correct scientific language when describing equilibrium shifts. Print slides 15-16 so the students can complete the exercise on the sheets rather than copying from the board. Answers animates onto the screen as you click when you are ready to review their learning.
Slides 18 - 20: students will be taught how changes in concentration, pressure and temperature affect the position of equilibrium
Slides 21-29: three questions which students can do on many white boards or as an independent practise task in their books about changes to conditions and equilibrium shifts. Answers are included with the resource. Please print slides 24-29 so that students can have the questions in front of them
Slide 30 – How does a catalyst affect the position of equilibrium?
Slide 31 – ALT (Applied Learning Task): a series of questions to test students’ understanding of the lesson. Answers animate on to the screen when you click
If you have a positive experience with the resource, please leave a positive review! This really helps promote our store! Thanks!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.