
How to construct a Born-Haber cycle for the lattice energy of an ionic compound? What is the difference between lattice enthalpy of formation and lattice enthalpy of dissociation? What is the definition of electron affinity and why is first electron affinity usually negative? All this and more covered in this comprehensive lesson with questions and answers! This is a Year 13 A level lesson for Edexcel International Unit 4 – WCH14, but it can also be used for all UK exam boards. All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question. The breakdown of the slides (which are best opened on Microsoft PowerPoint) is as follows:
Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.
Slide 2 - Lesson objectives (see thumbnail image)
Slide 3 – Hinge question: What gives us a measure of the strength of the covalent bonding in molecules? (Bond enthalpies is the answer)
Slide 4 – Hinge question: What gives us a measure of the strength of ionic bonding in ionic compounds? (Lattice energies is the answer)
Slide 5 – Definition of standard lattice energy (of formation) presented to students, with a few example equations
Slides 6 – 8 – Sample lattice energy data
Slide 9 – Inquiry question: Why is the lattice energy of magnesium chloride much larger than that of sodium chloride? Answer is explored on this slide
Slide 10 – Rules for when lattice energy will be more negative
Slides 11 – 13: Students introduced (or reintroduced) to definitions of atomisation, ionisation energy and electron affinity
Slides 14 – 18: Why is first electron affinity usually negative and second electron affinity always positive? This is explained here, alongside notable exceptions
Slide 19 - Mini plenary (Learning pit-stop). A set of questions for students to have some independent practice. The questions get progressively harder. Answers animate as you click!
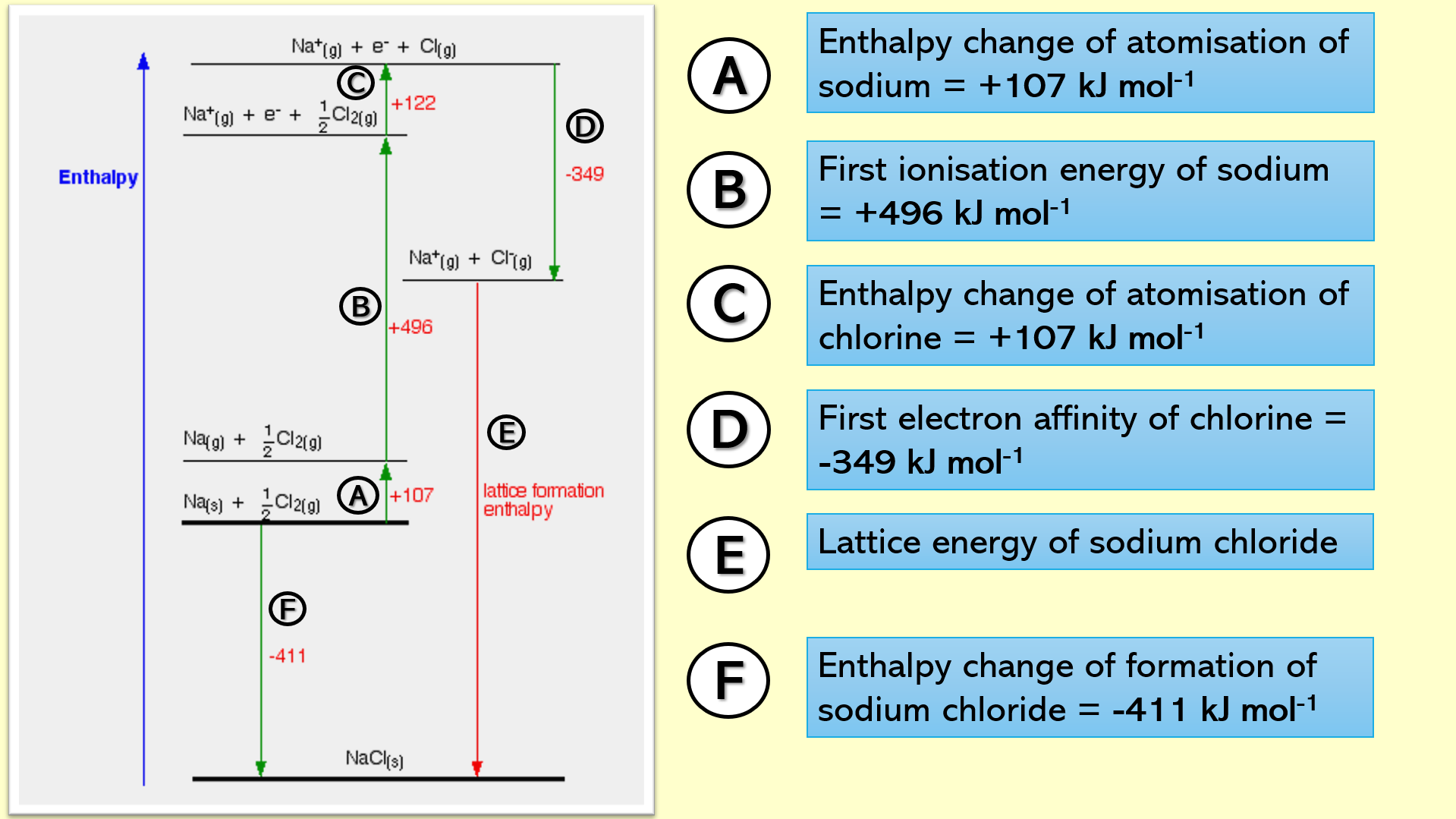
Slides 20 – 23: Students have the opportunity to label a Born-Haber cycle (which you will print for them) as you click, step-by-step
Slide 24 – Students use their Born-Haber cycle to calculate the lattice energy
Slides 25 – 29: Mini plenary (Learning pit-stop). A set of questions for students to have some independent practice. The questions get progressively harder. Answers animate as you click! Students must construct their own Born-Haber cycle based on the data provided and subsequently calculate the missing data value (second electron affinity)
Slides 30 - 36: Exam questions with mark scheme answers (included with the resource)
If you have a positive experience with the resource please leave a positive review! This really helps promote our store! Thanks!
Get this resource as part of a bundle and save up to 46%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Edexcel International A level Chemistry - lessons with questions and answers | Topic 12B | Lattice Energy
Bundle is comprised of 3 PowerPoint lessons covering Topic 12B – Lattice Energy for the Edexcel International A level Chemistry course, Unit 4 – WCH14, but it can be used for all UK exam boards which have a lattice energy unit. The PowerPoints are as follows: 1. Lattice Energy and Born-Haber Cycles 2. Experimental and Theoretical Lattice Energies 3. Enthalpy Changes of Hydration and Solution Each lesson is exactly that . . . a lesson. There is a starter slide, lesson objectives slide, introduction of new knowledge slides, worked examples and then opportunities for students to try independent practice through mini plenary tasks comprised of several questions of graded difficulties. Questions are interspersed through the lessons at regular intervals and always have answers that animate on to the screen when you click. THESE ARE NOT THE INFORMATION ONLY TYPE SLIDES THAT SOME PEOPLE SELL, which are useless for delivering a lesson. We are a team of teachers, and these lessons have been made by actual Chemistry teachers who currently teach in secondary schools in England. We know exactly what you need, trust me. Every lesson has exam questions included as word documents which were made using Exam wizard. The mark scheme is at the end of each exam question and the answers have been screen shotted and pasted into the PowerPoint at the end. All the slides in every lesson are fully animated and include answers to every mini plenary question and exam question. If you have a positive experience with the bundle, please leave a positive review! This really helps promote our store!
Edexcel International A level Chemistry - lessons with questions and answers | Topic 12 | Entropy and Energetics
Bundle is comprised of 6 PowerPoint lessons covering Topic 12 – Entropy and Energetics for the Edexcel International A level Chemistry course, Unit 4 – WCH14, but it can be used for all UK exam boards which have Entropy and Energetics units. The PowerPoints are as follows: 1. Entropy Introduction 2. Total Entropy Change 3. Understanding Entropy Changes 4. Lattice Energy and Born-Haber Cycles 5. Experimental and Theoretical Lattice Energies 6. Enthalpy Changes of Hydration and Solution Each lesson is exactly that . . . a lesson. There is a starter slide, lesson objectives slide, introduction of new knowledge slides, worked examples and then opportunities for students to try independent practice through mini plenary tasks comprised of several questions of graded difficulties. Questions are interspersed through the lessons at regular intervals and always have answers that animate on to the screen when you click. THESE ARE NOT THE INFORMATION ONLY TYPE SLIDES THAT SOME PEOPLE SELL, which are useless for delivering a lesson. We are a team of teachers, and these lessons have been made by actual Chemistry teachers who currently teach in secondary schools in England. We know exactly what you need, trust me. Every lesson has exam questions included as word documents which were made using Exam wizard. The mark scheme is at the end of each exam question and the answers have been screen shotted and pasted into the PowerPoint at the end. All the slides in every lesson are fully animated and include answers to every mini plenary question and exam question. If you have a positive experience with the bundle, please leave a positive review! This really helps promote our store!
Something went wrong, please try again later.
very useful. i've been out of the classroom for a while, so need as much support like this. thanks!
to let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.